fuel
cells work
An electrochemical
energy conversion device to convert the chemicals hydrogen and oxygen convert into water, and in the process it produces
electricity.Battery the other electrochemical device that we are all familiar.A
battery has all of its chemical stored inside, and it converts those chemicals
into electric current. This means that a battery eventually “goes dead” and you
either throw it away or recharge it.
For fuel cell
Chemicals constantly
flow into the cell so it n
never goes dead.
As long as there
is a flow of chemicals into the cell.
The electricity
flows out of the cell
The most fuel
cells in use today use hydrogen and oxygen as the chemicals.
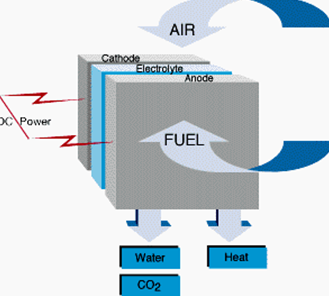
It consists of
three components a cathode, an anode, and an electrolyte sandwiched between the
two. Oxygen from the air flows through the cathode. A fuel gas containing
hydrogen, such as methane, flows past the anode. Negatively charged oxygen ions
migrate through the electrolyte membrane react with hydrogen to from water. The
reacts with the methane fuel to form hydrogen and carbon dioxide.
Cool fuel cells
Fuel cells promise to be the environmentally friendly
power source of the future.
But some types
run too hot to be practical NASA-funded research may have a solution.
Basic configuration
PEMFC
IF YOU HAVE ANY DOUTS
FEEL FREE TO COMMENT AT HERE.....!!!